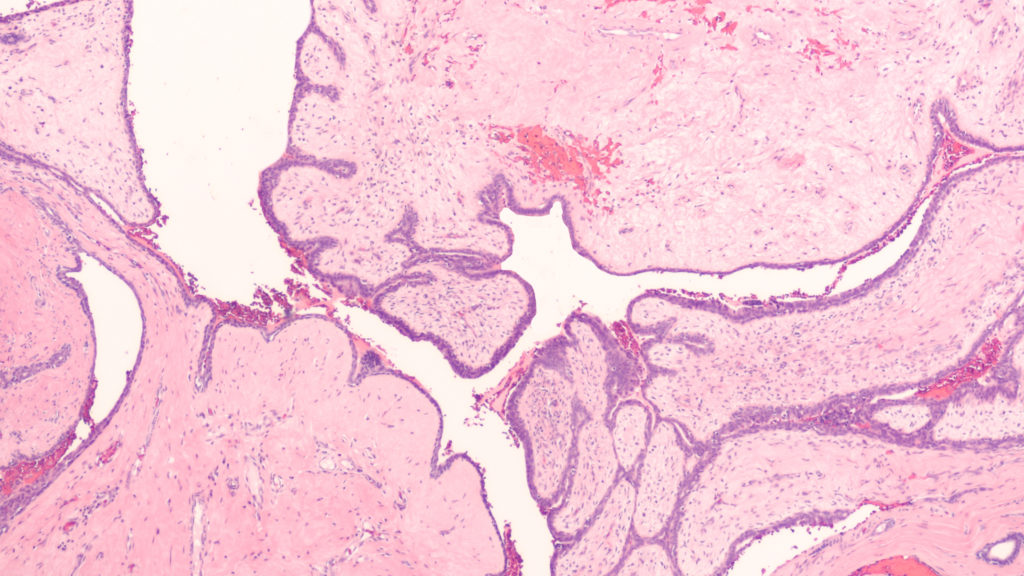
In a new editorial, researchers discuss a study using their team’s new genetically engineered mouse (GEM) model to assess PLK1 as a driver of oncogenic transformation.

On the bright side, polo-like kinase 1 (PLK1) is considered a master regulator of the ever-important cell cycle. On the dark side, PLK1 expression (at both the mRNA and protein level) has shown to be upregulated in tumor cells, suggesting that PLK1 may also contribute to tumorigenesis. Despite this direct association, researchers studying the role of PLK1 in cancer have encountered a problem: a lack of appropriate animal models for experimentation.
“Even though studies have suggested that PLK1 contributes to tumorigenesis, the ability of PLK1 to drive oncogenic transformation on its own in vivo was still questionable due to a lack of sophisticated animal models for experimentation [18, 19].”
This problem may have been solved in 2021. In a new editorial paper, researchers Lilia Gheghiani and Zheng Fu from Virginia Commonwealth University discuss a recent study using their team’s new genetically engineered mouse (GEM) model to assess the ability of PLK1 to be a sole driver of oncogenic transformation in vivo. Their editorial was published in Oncotarget’s Volume 14 on June 27, 2023, and entitled, “The dark side of PLK1: Implications for cancer and genomic instability.”
PLK1 in Tumorigenesis
“To address this important scientific question, we generated a new genetically engineered mouse (GEM) model using the CAGGS (cytomegalovirus (CMV) early enhancer/chicken β-actin) promoter to drive exogenous PLK1 expression, allowing its ubiquitous and robust gene expression in transgenic mice [20].”
In an effort to determine if PLK1 overexpression causes tumors, the researchers created a new GEM mouse model that expresses high levels of PLK1. These high levels caused various types of spontaneous tumors. The increased PLK1 levels caused defects in cell division and resulted in abnormal numbers of centrosomes and compromised cell cycle checkpoints. This allowed for the accumulation of chromosomal instability, leading to abnormal numbers of chromosomes and tumor formation. In human cancers, higher PLK1 expression was associated with an increase in genome-wide copy number alterations. Their study provides evidence that abnormal PLK1 expression can trigger chromosomal instability and tumor formation, suggesting potential therapeutic opportunities for cancers with chromosomal instability.
“In summary, this study provides a novel GEM model that recapitulates the increased PLK1 expression observed in many human cancers and demonstrates that PLK1 overexpression drives spontaneous tumor formation in multiples organs in mouse, revealing the dark side of PLK1 as a potent proto-oncogene.”
Conclusions
In conclusion, the limitations of previous studies on PLK1 and its role in cancer have been partially addressed by the development of the new GEM model created by these researchers. This model allowed the team to examine PLK1’s ability to drive oncogenic transformation in vivo. Their study demonstrates that overexpression of PLK1 leads to the formation of spontaneous tumors in multiple organs, highlighting the dark side of PLK1 as a potent proto-oncogene. The findings of this study provide valuable insights into the role of PLK1 in tumorigenesis and suggest potential therapeutic opportunities for cancers associated with chromosomal instability. This breakthrough in animal models opens up new avenues for further research in understanding the mechanisms underlying PLK1-related tumorigenesis and developing targeted therapies to combat cancer.
“Alternative therapeutic strategies, such as co-delivery systems using nanoparticles or combination therapies, are under development in order to enhance the efficacy of PLK1 inhibition [25–28]. With expanding discoveries of PLK1 function and mechanisms of action, we hope that PLK1-targeted therapies will soon join the frontlines in the fight against cancer.”
Click here to read the full editorial paper in Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com. Oncotarget is indexed/archived on MEDLINE / PMC / PubMed.
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.