In a recent Oncotarget editorial, researchers discuss Kras and the canonical Wnt pathway in biliary tract cancers, and potential theraputic strategies using these targets.
The extrahepatic biliary system is a network of tubes and ducts that carry bile from the liver to the small intestine, where it helps digest fats. Biliary tract cancers, including gallbladder cancer and cholangiocarcinoma, are rare but aggressive cancers that arise from this system. Understanding the molecular mechanisms that drive these cancers is crucial for developing effective therapies.
“Despite advances in diagnosis and therapy, 5-year survival rate of biliary cancer is only 5% to 15% [7, 8].”
In a new editorial, researchers Munemasa Nagao, Akihisa Fukuda and Hiroshi Seno from Kyoto University Graduate School of Medicine discuss the latest research on the role of Kras and the canonical Wnt pathway in the development of biliary tract cancers. On January 26, 2023, their paper was published in Oncotarget’s Volume 14, entitled, “The role of Kras and canonical Wnt pathways for tumorigenesis of extrahepatic biliary system.”
Kras and The Canonical Wnt Pathway in Biliary Tract Cancers
Kras is a gene that plays a key role in regulating cell growth and division. Mutations in Kras are common in many types of cancer, including biliary tract cancers. The authors of this editorial note that recent studies have shown that Kras mutations are relatively frequent in biliary cancers. These mutations activate the Kras protein, leading to uncontrolled cell growth and division.
The canonical Wnt pathway is another molecular pathway that has been implicated in cancer development. The Wnt pathway helps regulate cell growth and division during embryonic development and in adult tissues. Abnormal activation of the Wnt pathway has been linked to several types of cancer, including colon cancer and liver cancer. Recent studies have shown that the canonical Wnt pathway is also activated in biliary tract cancers.
“However, the role of the KRAS and WNT pathways in biliary tumorigenesis remained unclear.”
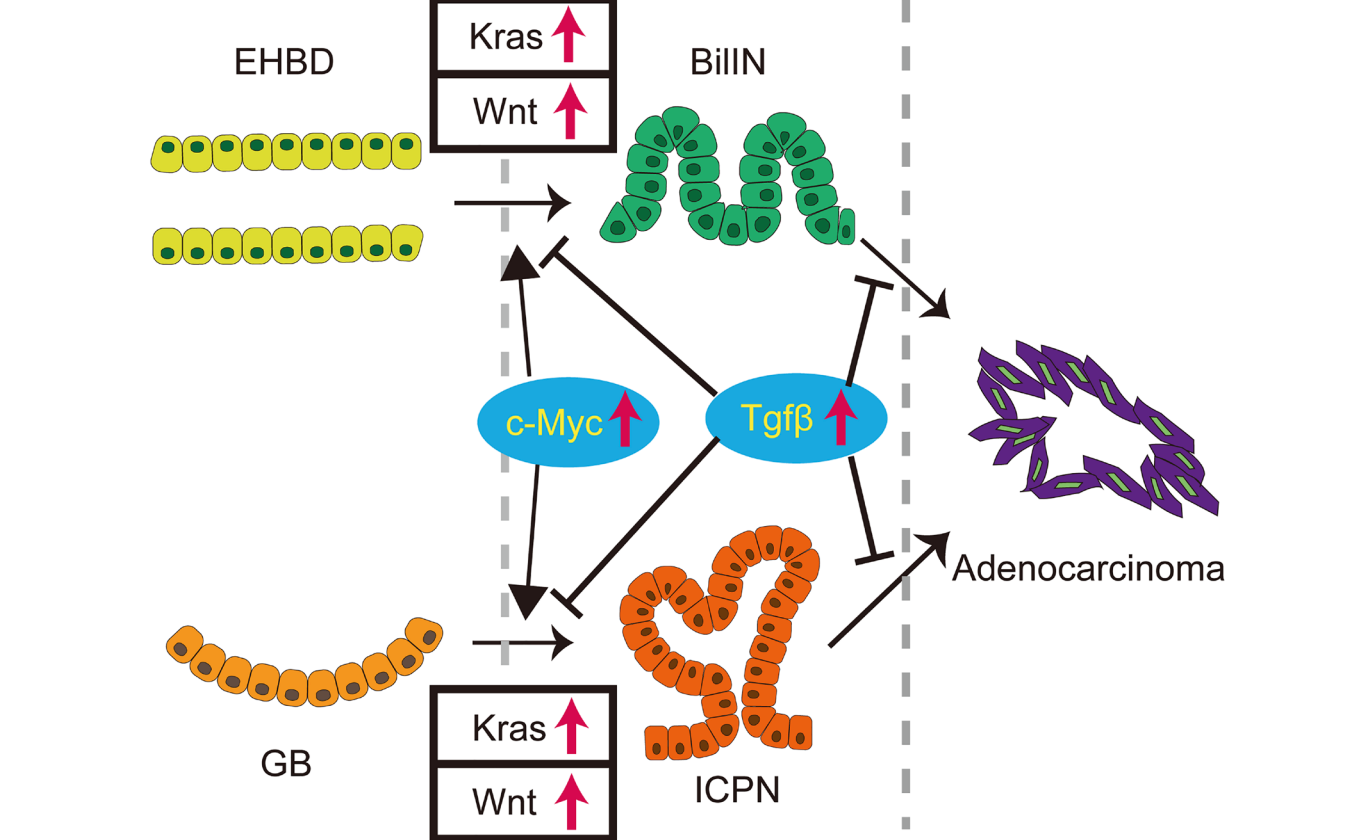
A protein called beta-catenin, which is a key component of the Wnt pathway, is often overexpressed in biliary tract cancers. This leads to the activation of downstream target genes that promote cell growth and division. The researchers also discussed results from their 2022 study investigating the role of the Kras and canonical Wnt pathways in the tumorigenesis of the extrahepatic biliary system using a genetically engineered mouse (GEM) model.
“In summary, concurrent activation of the Kras and Wnt pathways in the extrahepatic biliary system induced ICPN and BilIN, which can progress to biliary cancer (Figure 1). This study provides the first novel GEM that recapitulates human ICPN and BilIN, establishing them precancerous lesions. This work shows how dysregulation of canonical cell growth pathways drives precursors to biliary cancers and identifies several molecular vulnerabilities as potential therapeutic targets in these precursors to prevent oncogenic progression.”
Potential Therapeutic Strategies for Biliary Tract Cancers
The researchers go on to discuss the concurrent activation, or crosstalk, between Kras and these pathways in biliary tract cancers. They note that several studies have shown that Kras mutations can activate the Wnt pathway, leading to even more aggressive cancer growth. This suggests that targeting both pathways may be necessary for effective therapy.
Potential therapeutic strategies targeting Kras and Wnt pathways in biliary tract cancers were discussed in this editorial. Several drugs that target these pathways are currently in development, and some are already being tested in clinical trials. The authors suggest that combining these drugs with chemotherapy or other targeted therapies may be a promising approach for treating biliary tract cancers.
“To develop novel preventive and therapeutic approaches for extrahepatic biliary cancer, it is also important to clarify the role of other altered genes by using a GEM model and/or human samples.”
Conclusion
Overall, this editorial provides a comprehensive overview of the latest research on the molecular mechanisms underlying biliary tract cancers. Their discussion of the role of Kras and the canonical Wnt pathway highlights the importance of understanding these pathways for developing effective therapies. The potential for combination therapies targeting both Kras and the canonical Wnt pathway is particularly intriguing, and could offer new hope for patients with these aggressive cancers.
Withstanding, it is important to note that more research is needed before these ideas can be translated into clinical practice. The authors themselves acknowledge that the development of effective targeted therapies for biliary tract cancers is still in its early stages. However, with continued research and collaboration, it is possible that new treatments will emerge that can improve the prognosis for patients with these challenging cancers.
Click here to read the full editorial published in Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com. Oncotarget is indexed/archived on MEDLINE / PMC / PubMed.
For media inquiries, please contact media@impactjournals.com.