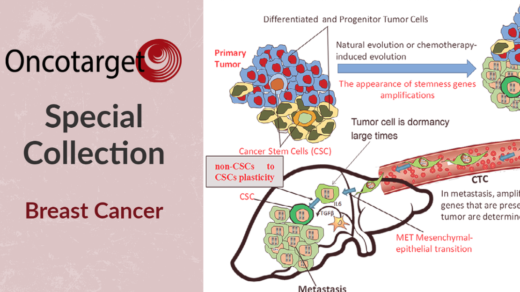
“Cancer dormancy, followed by recurrence remains a poorly understood phenomenon in both cancer biology and oncology.”
Cancer dormancy is a phenomenon in which, after treatment, residual cancer cells remain inactive in the body for months or even years. During this time, patients often show no signs of the disease. These dormant cells can unpredictably reawaken, leading to tumor recurrence—a significant challenge in cancer treatment. Despite progress in cancer research, the factors that control dormancy and subsequent reactivation remain poorly understood. Identifying these factors and understanding how cancer cells dormancy and reactivation occur could be crucial to preventing cancer recurrence. This question was the focus of a recent study titled “Initiation of Tumor Dormancy by the Lymphovascular Embolus,” published in Oncotarget Volume 15, on October 11, 2024. In this blog, we will look at the key findings and implications of this important work.
The Study: Investigating Dormancy in Breast Cancer Tumors
This study, led by Yin Ye, Justin Wang, Michael G. Izban, Billy R. Ballard, and Sanford H. Barsky from Meharry Medical College and Scripps Mercy Hospital, aimed to investigate the origins of cancer dormancy, an often overlooked aspect of cancer progression, focusing specifically on breast cancer.
Using various breast cancer study models—such as patient-derived mice, spheroids, and cell lines—the researchers investigated how dormancy might start within small clusters of cells known as lymphovascular emboli, which detach from the primary tumor. These clusters can travel through the bloodstream or lymphatic system, settle in distant organs, and remain inactive until conditions change, triggering their reactivation and growth. To further validate their findings, the team analyzed tissue samples using tissue microarrays, allowing them to observe dormancy indicators directly in human breast cancer cases.
The Challenge: Elusive Dormant Cancer Cells
Dormant cancer cells pose a unique challenge because they grow slowly and often evade immune system detection, making them difficult to target with conventional treatments. These cancer cells typically exist as small, inactive clusters called micrometastases, which can later transition back into an active state and lead to tumor recurrence. Preventing this recurrence requires understanding how these cells “decide” to stay dormant or reawaken.
Dormancy periods vary depending on the type of cancer and the individual patient, making it even more important to pinpoint the factors that influence cancer cell dormancy and reactivation. Identifying these factors could transform our approach to cancer treatment.
The Results: A Breakthrough in Cancer Dormancy Mechanisms
The team found that cancer cells within lymphovascular emboli may enter dormancy through a reduction in key cellular activities. Two important players in this process are mTOR signaling and E-cadherin proteolysis. mTOR is a cellular pathway involved in regulating cell growth and metabolism, which, when reduced, slows the cell’s activity to a near standstill, facilitating dormancy. Meanwhile, E-cadherin, a protein that helps cells stick together, undergoes a process called proteolysis, or breakdown, through enzymes like calpain 2. This proteolysis further stabilizes the dormant state, keeping the cells inactive until reactivation signals arise. The researchers also discovered that the PI3K signaling pathway influences these dormancy-associated changes in mTOR and E-cadherin. Together, these signaling modifications within the three-dimensional structure of lymphovascular emboli reveal how dormant cancer cells persist in a state of low activity until conditions favor their reactivation.
The Potential: Toward New Treatments for Preventing Cancer Recurrence
This study demonstrates the potential for targeted interventions to prevent dormant cells from reawakening. Developing therapies that act on mTOR and E-cadherin pathways might provide cancer patients with a new line of defense against recurrence, especially in cancers prone to prolonged dormancy, such as breast cancer. Although further research is needed to determine the exact clinical applications, these findings provide a promising roadmap for future treatment innovations.
Conclusion
This work represents a significant step forward in our understanding of cancer dormancy and recurrence. By uncovering the mechanisms behind cancer cell dormancy, this research brings us closer to a future where cancer recurrence can be controlled—or even prevented entirely. While more studies are necessary to explore the broader implications for other types of cancer, this study highlights a critical aspect of cancer biology and offers hope for more effective and targeted treatments in the near future.
Click here to read the full research paper in Oncotarget.
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.